FAQ
Q1 What is hydroxyapatite?
Hydroxyapatite is a calcium phosphate occurring naturally in the human body, where it makes up the hard tissues of tooth and bone. It is edible, highly biocompatible, and used widely in medical and dental applications such as bone repair and coatings to improve the acceptability of orthopedic implants. Apatites have the unique ability to maintain their chemical identity and crystal structure even if some of the ions in their chemical formula are displaced, and this means they can exist in a wide range of forms with very different properties, depending on the method of synthesis used.
(more information)
Q2 What is SANGI's ingredient nano<mHAP>?
SANGI's toothpaste ingredient nano<mHAP>, first synthesized in the 1970s, is a type of hydroxyapatite developed specially for remineralization of tooth enamel. It was approved by the Japanese government as an anti-caries agent in 1993 and given the name 'Medical Hydroxyapatite' to distinguish it from other types of hydroxyapatite such as dental abrasives.
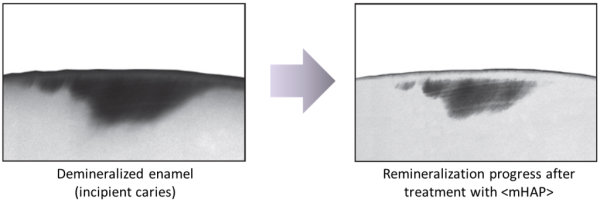
Q3 How can teeth be 'remineralized'?
Bones contain cells known as osteoblasts which are constantly renewing and replacing our bone tissue. But no such cells exist in tooth enamel, once it is formed. Emerging teeth are immediately subject to wear and tear, and to demineralizing attack by acids from foods and from plaque bacteria. What isn't widely recognized, however, is that saliva plays a key role in protecting our teeth, first by neutralizing the acids, and then by supplying calcium and phosphate ions, the building blocks of hydroxyapatite, to replace lost mineral. Demineralization and remineralization take place constantly at the tooth surface, and if demineralization gets the upper hand, incipient caries and eventually fully fledged caries requiring professional treatment will occur. So although our tooth enamel can't renew itself from within, its mineral density can be and is constantly being supported by external supply of mineral.
Q4 What are the functions of SANGI's nano<mHAP> ingredient?
In approving SANGI's original hydroxyapatite as an anti-caries agent in 1993, Japan's then Ministry of Health recognized three functions of the ingredient as protecting against tooth decay, namely.
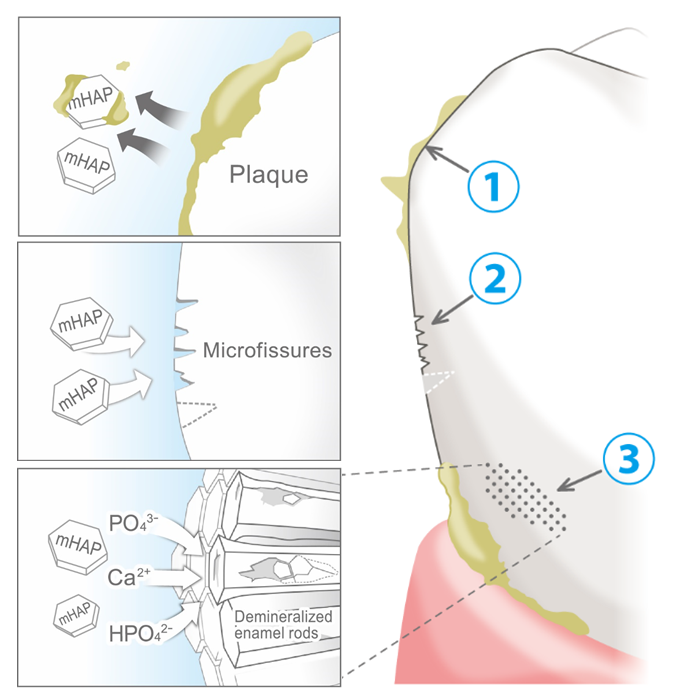
1 adhering to and helping remove plaque and plaque bacteria,
SANGI's nano<mHAP> also relieves hypersensitivity, by sealing and forming a coating over exposed dentinal tubules, protecting against access of external stimuli like heat and cold to the dental nerves.

Another function is that, by restoring surface smoothness and subsurface mineral density to the enamel, SANGI's <mHAP> can steadily restore the natural whiteness of the user's teeth.
Q5 How can nano<mHAP> collect and help remove bacteria and at the same time remineralize tooth enamel?
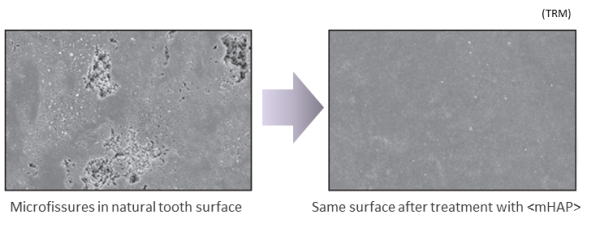
Of the total amount of nano<mHAP> in the mouth during toothbrushing, most will be rinsed out after brushing, carrying with it a lot of bacteria and plaque fragments that collected on its nanoparticles during brushing, leaving the mouth feeling extremely clean. But a portion of the <mHAP> nanoparticles will be incorporated on and into the tooth surface, filling and repairing surface microfissures and helping to remineralize mineral-depleted areas below the enamel surface, i.e. incipient caries lesions, the weak spots from which decay starts. And even after rinsing, a small fraction will remain in the saliva, increasing its calcium and phosphate content, and supporting its remineralizing role. Which is why we advise users to brush gently for several minutes to allow maximum uptake, and to rinse only lightly after brushing, for residual effect.
Q6 Fluoride also remineralizes, and it makes teeth less vulnerable to acids, so isn't it better than hydroxyapatite?
Fluoride doesn't actually 'remineralize' teeth - because unlike saliva or nano<mHAP>, it contains no mineral. It's actually a highly active element - the most active on the periodic table - and it acts rather like a catalyst to promote remineralization by calcium and phosphate ions from the surrounding environment, e.g. from saliva or other sources, forming a new substance, fluorapatite or fluoridated apatite, on the surface of the teeth. This substance is more resistant to acids than the enamel's natural hydroxyapatite, so this is one benefit of fluoride over hydroxyapatite. On the other hand, by forming a hard coating on the tooth surface, fluoride can actually block further entry of remineralizing agents into the enamel, so that demineralized areas below the tooth surface (so-called 'white spot' lesions) can become permanently enclosed. In contrast, nano-hydroxyapatite, over time, has been shown to penetrate to the depth of such lesions and eliminate white spots, whereas fluoride does not.
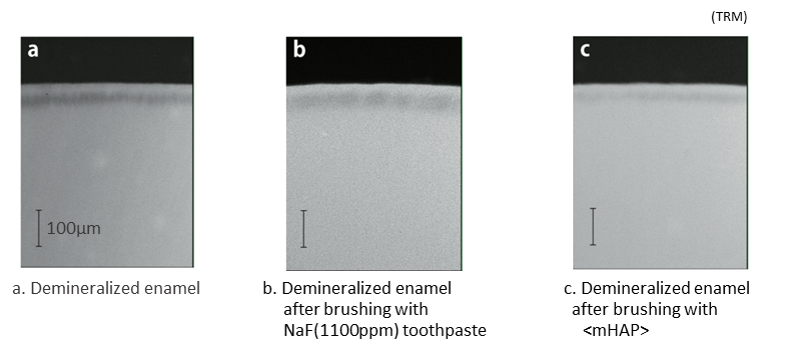
Q7 Why doesn't SANGI have fluoride in its toothpastes?
This is mainly for three reasons: (1) because SANGI's nano<mHAP> is almost the same substance as tooth enamel and itself directly remineralizes the enamel; (2) because the anti-caries benefits are equivalent; and (3) because some people prefer toothpaste without fluoride, especially for young children. We offer consumers an alternative choice.
Q8 Can we use SANGI's toothpastes in combination with a fluoride toothpaste or other fluoridation agents?
This isn't really necessary, but some people like to alternate between using a fluoride toothpaste and SANGI's nano<mHAP> hydroxyapatite toothpaste, or to use nano<mHAP> toothpaste in combination with a fluoride rinse. Perhaps an ideal combination might be to use SANGI's nano<mHAP> toothpaste in a country where you have fluoride in the water system. That way you would have the necessary remineralizing component – nano<mHAP> being almost the same substance as the teeth – plus fluoride acting as a catalyst to speed uptake of the hydroxyapatite into the teeth, at the same time strengthening the enamel's acid resistance. But SANGI's early research suggests no such synergy exists, and if fluoride forms a hard coating on the enamel surface, this may block entry of the remineralizing ions and nanoparticles deeper into the teeth, with the result that nano<mHAP>'s ability to remove 'white spots' by remineralizing to the depth of the lesions may be compromised.
Q9 How can there be so many different forms of hydroxyapatite with the same chemical formula? For example, how can some be abrasive and others not?
The compounds known as apatites were given their name by a German scientist, Abraham Gottlob Werner, who first discovered them in the late 18th century. He chose the name 'apatite,' from the Greek word 'apato,' meaning 'elusive,' because he found that apatites do not behave like other chemical compounds. They can sustain significant loss or replacement of ions from their molecular structure without losing their common chemical identity, and can have widely differing characteristics and functions, depending on how they are made. SANGI specializes in different forms of hydroxyapatite, developing them for use in a wide range of applications, including other than for dental use, and nano<mHAP> has been developed specifically to be without abrasivity, for superior remineralization and bacterial adsorption use.
Q10 What exactly is the difference between SANGI’s nano<mHAP> and other hydroxyapatites used in toothpastes?
That is something we don't know. It’s not possible to extract and characterize the hydroxyapatite contained in a toothpaste, i.e. to examine its specifications such as morphology and particle size. One can only gain an idea of the volume contained. In Japan, most of the hydroxyapatite used in toothpaste is classed as either abrasive, adsorbent or excipient (i.e. used as a base material), and that may possibly be the case elsewhere. SANGI's nano<mHAP> comprises pure hydroxyapatite, at extremely high purity, whereas some other hydroxyapatites used in toothpaste contain metal or protein additives, or may be present in conjunction with fluoride compounds. One useful guide, however, when comparing ingredients, is to check the manufacturer's original research - whether specific studies have been carried out to substantiate its claims for its particular ingredient. What we can say about nano<mHAP>, in this regard, is that its enamel-restorative and bacteria-reducing properties have been scientifically and clinically demonstrated over the last four decades, and in a number of countries, including Japan and Canada, its status as an anti-caries agent has been government approved.
Q11 From what age can SANGI's nano<mHAP> toothpaste be used?
In Japan there is no age restriction on the use of nano<mHAP>-containing toothpaste. SANGI's economy- sized APAGARD M-Plus is mildly mint-flavored and commonly purchased for whole family use. However we have developed several toothpastes especially for children, including a gel for infants, with a fruity flavoring, added xylitol, and less ingredients and foaming agent than in adult pastes. Nano<mHAP> is quite safe if swallowed, but for very small children, who can’t yet spit out the paste, we recommend parental supervision and use of a swab or gauze to wipe away toothpaste remaining in the mouth after brushing.
Q12 Is nano<mHAP> safe?
Despite the huge spread of nanomaterials in industry, medicine and our daily lives over the last five decades, consensus has never been reached internationally on safety assessment or regulatory standards. Even the question of what constitutes a nanomaterial is still open to debate. As a result, in some circles there has been a certain phobia regarding nanomaterials, leading to the view that, regardless of the material, anything nano-scale must by definition be unsafe. Hydroxyapatite, however, exists naturally in our bodies at nano-scale. When synthesized in a test tube by simple chemical reaction its primary particles are nanosized. It is known for its biocompatibility, and approved widely for use in the human body, in applications such as catheter coatings and bone fillers. Hydroxyapatite can be eaten, and provides a rich source of calcium and phosphate in foods.
SANGI's nano<mHAP> is safe for use in toothpaste because, even if swallowed, its nanoparticles will be immediately broken down by stomach acids. Studies indicate that its nanoparticles will not penetrate into the deeper tissues via the oral mucosa, which like our skin, provides a multilayered barrier against the entry of foreign bodies and pathogens. SANGI's toothpastes are not only safety-approved, but have been marketed in Japan for over 4o years (to date, over 150 million tubes sold), during which time no adverse event or claim of serious bodily harm was ever reported, while complaints of irritation to the oral tissues (possibly from other ingredients in the toothpastes) averaged 1.2ppm per year. We assure you with confidence that SANGI's nano<mHAP> ingredient is extremely safe.
Q13 What is the RDA of SANGI's toothpastes?
The validity and importance of RDA measurement remains a subject of some contention, and in Japan it is rarely referred to. However SANGI had its toothpastes for the European market tested by a highly qualified Swiss authority. The RDA of our main series, APAGARD M-plus, SMOKIN', PREMIO, and APADENT TOTAL CARE, PERIO and SENSITIVE, all of which contain dental-grade calcium phosphate dibasic (DCPD) as a base material, is each around 80, while APADENT KIDS, which is silica-based, has an RDA below 10. Nano<mHAP> itself, being almost identical in substance and hardness to tooth enamel, and in nanoparticle form, is not abrasive, but rather a remineralizing agent, which means that although the RDA of our adult-use brands may be slightly higher than that reported for some other toothpastes, any abrasivity that might result from the inclusion of DCPD would be more than compensated by the mineral replacement function of the nano<mHAP> active ingredient.
Q14 What is the volume of SANGI's toothpastes in ml?
Expressed in milliliters, the volume of SANGI's toothpastes would be as follows:
APAGARD PREMIO and SMOKIN' (100g): 69ml
APAGARD M-plus (125g): 86ml
APADENT (all three types, 60g): 41ml
Q15 Do SANGI's toothpastes contain gluten or GMO?
SANGI’s toothpastes contain no gluten or GMO (genetically modified organisms).
Q16 What are the main differences between APAGARD and APADENT?
All SANGI’s toothpastes contain and offer the benefits of our ingredient nano<mHAP> – namely plaque control, mineral restoration, anti-caries, anti-hypersensitivity and natural whiteness. But for historic reasons, in the case of APAGARD we focus on nano<mHAP>'s natural whiteness and anti-caries benefits, and the extra gloss provided by the addition of a pearl protein derivative, conchiolin. In the case of APADENT we focus more on all-round oral health, including periodondontal care, freshness of breath, and anti-hypersensitivity, for example providing polyphenols for the gums, or potassium nitrate to block transmission of stimuli to the nerves. This difference allows us to address the concerns of different groups of users, and to more closely meet their needs - for example, younger women and people more concerned with beauty, in the case of APAGARD, and those more concerned with general health, such as children and the older generation in the case of APADENT.
Q17 Where can I buy SANGI's toothpaste?
SANGI's toothpastes are available in different retail outlets and on-line shops in different countries.
For details of our distribution partners nearest to your location, click here.